A Historical Moment For Medical Advances
Eisai and Biogen’s new drug, lecanemab, has proven to slow down the cognitive decline of Alzheimer’s patients.
October 10, 2022
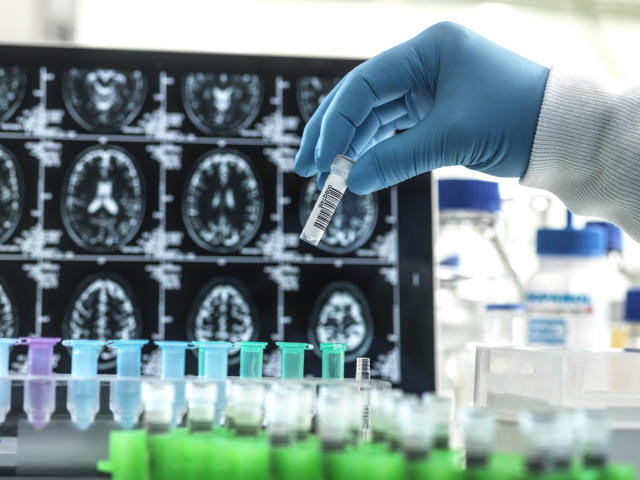
As of now, there is no permanent cure for Alzheimer’s. The disease is known to attack the brain, causing memory loss, communication difficulties, and negatively impacting the performance of basic tasks. Currently, scientists do not completely understand the disease, making it harder to treat it. The uprising of the lecanemab drug has shown the most progress in helping fight the disease. Last year, Biogen introduced a drug called Aduhelm, which failed to treat Alzheimer’s. Due to a series of failures, people are hesitant to move forward quickly with new treatments. Yet, with the progress being shown by the new drug, people are hoping this is a big step toward advancements in treating Alzheimer’s.
Recently, the pharmaceutical companies Biogen and Eisai have discovered a new treatment for Alzheimer’s after a series of trials and errors. The possible new drug, lecanemab, has been deemed a historical moment as it is the first Alzheimer’s medication proven to be successful in recent years. It is also the first time a drug has been closely linked to slowing a disease’s trajectory. Medical regulators will review the results of the new drug for it to be approved for the market (Sky News). Some people may be skeptical to trust the new medicine, but it has been tested on about 1,800 patients while being compared to patients on placebo treatment. The cognitive decline of the patients given the drug slowed down by 27% after 18 months. Patients were given infusions of the drug twice a week. Results concluded that it reduced toxic plaques in the brain and slowed down a cognitive decline (The Guardian). However, some patients taking lecanemab experienced brain swelling or bleeding, but these side effects are lower than any other test drug for Alzheimer’s.
Currently, Eisai is seeking approval from the Food and Drug Administration as the drug still requires further research and tests. The company hopes to pass the drug because it proves to affect the disease’s biological track. Even though there is uncertainty about the lecanemab’s full effects, studies have shown that it helps Alzheimer’s patients. With approval, this could earn the company billions of dollars, and more importantly, it could help better the lives of patients with dementia. The company’s breakthrough discovery has led share prices to surge. (The New York Times).
the medical advancements we’re seeing now, such as vaccines and new treatments, make me hopeful for the future of medicine. Maybe one of the current fatal diseases and illnesses will be treatable — Leia Fidel
Over generations, new discoveries like this can be a major stride in medical advancements. We can better understand diseases and learn how to stop them. Leia Fidel (10) adds her thoughts on this, saying, “the medical advancements we’re seeing now, such as vaccines and new treatments, make me hopeful for the future of medicine. Maybe one of the current fatal diseases and illnesses will be treatable.” The successful research is giving hope to many others that progress can be made in curing diseases. Since many people are affected by diseases like Alzheimer’s, a seemingly incurable disease, recent discoveries are bringing hope to people.