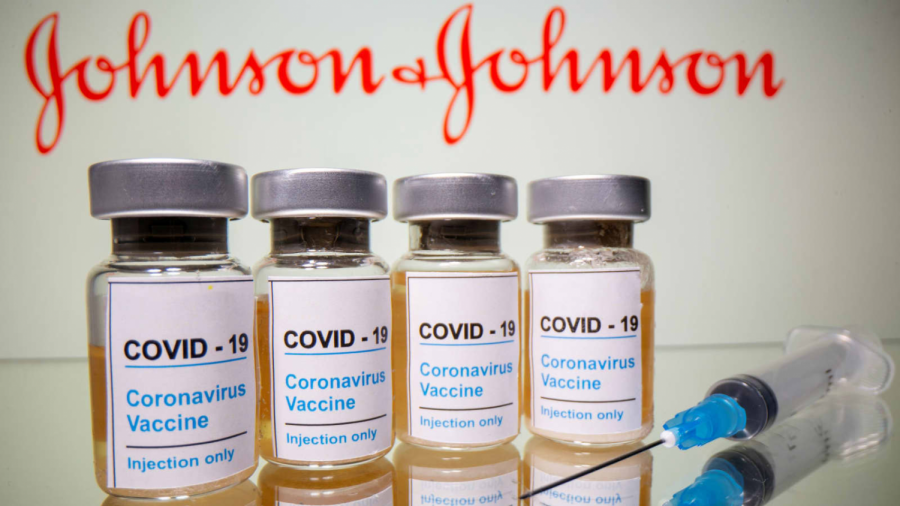
The Johnson & Johnson Vaccine
Johnson and Johnson’s Janssen vaccine has been approved by the FDA and is starting to be administered to the public.
March 14, 2021
With two vaccines, the Pfizer and Moderna vaccines, being distributed, everyone in the United States is awaiting for more vaccines to be approved to hasten the vaccination process. Thankfully, on February 27, 2021, the US Food and Drug Association (FDA) issued emergency authorization of Johnson & Johnson’s Janssen vaccine (fda.gov).
The FDA unanimously decided to approve the vaccine after a Phase 3 ENSEMBLE which indicated that their vaccine was 85% effective and displayed protection against COVID-19 hospitalization and death in all regions that they were tested in. The phase 3 ENSEMBLE Study was a randomized and placebo-controlled study on only individuals 18 years or older, and this study spread across 8 countries and 3 continents with 43,783 participants. This vaccine is effective after 28 days (jnj.com) The side effects of this vaccine include pain, redness, and swelling where the shot was administered, and throughout the body, individuals who received this shot can experience tiredness, headache, muscle pain, chills, fever, and nausea. Usually, these side effects start a day or two after receiving the vaccine and diminish within a few days (cdc.gov).
Unlike the Pfizer and Moderna vaccine, which uses messenger RNA to create the virus’s spike protein, this vaccine uses DNA that is encapsulated in an inactive adenovirus, which is the virus that can cause the common cold, that can not replicate in the body, and once the cell can create a spike protein found on coronavirus, the immune system responds leading to protection if the recipient is exposed to COVID-19. Besides, the Johnson & Johnson Vaccine only requires one dose, and John Wherry, the director of immunology at the University of Pennsylvania, thinks that “in terms of one-shot versus two shots, you can start thinking about underserved communities where it may be harder to follow up and ask people to come back for second shots.” Also, the Johnson & Johnson vaccine can be kept at normal refrigerator temperatures which is extremely helpful for the storage and distribution of the vaccine since they can be held in rural medical hospitals that do not have access or the money for the immensely cold refrigerators the Pfizer and Moderna vaccines need (whyy.org).
However, although many people and groups are overjoyed about the vaccine’s approval, Catholics and pro-lifers are strongly against this vaccine. Multiple bishops, clergy members, and pro-life organizations are condemning this vaccine because aborted fetuses were used to develop it. For instance, in New York, the Roman Catholic Diocese of Rockville Centre released a statement regarding the vaccine: “On moral grounds related to their connection to the evil of abortion, it is recommended that of the alternatives available now or very soon, the Pfizer and Moderna vaccines are preferred to the Johnson & Johnson and AstraZeneca vaccines” (newsday.com).
Nonetheless, after a year stuck in this pandemic, most United States citizens are elated about the news of this vaccine wanting their lives to return to normalcy. To hasten the distribution of the vaccine, the Biden Administration made a manufacturing collaboration with Merck and Johnson & Johnson, two of the largest healthcare and pharmaceutical companies, to expand the production of the Johnson & Johnson Vaccine. Also, President Biden has invoked the Defense Protection Act to facilitate materials in the production of the vaccine and directed the Department of Defense to grant daily logistical support to Johnson & Johnson’s vaccine efforts. With the support of the Biden Administration, Johnson & Johnson has begun operating facilities open 24/7 to maximize production output and believes that they will be able to deliver 100 million doses of the vaccine by the end of May (hhs.gov).
The Johnson & Johnson vaccine is a beacon of hope to those waiting to see their families and friends again without fear of contracting or giving COVID-19 to their loved ones. With three effective vaccines out, Lorenzo Artulo (11) is “hopeful that [his] life will return to somewhat normal soon.” While these companies continue to work hard to administer the vaccines to as many people as possible, many other companies are conducting trials to see if their vaccine candidate is effective as well. As more vaccines continue to come out and more people get vaccinated, a life without the pandemic is becoming more of a reality.








































Fiona Salisbury • Mar 19, 2021 at 9:03 PM
This article was super informative! Hopefully, now that the Johnson and Johnson vaccine is out, more people will be able to vaccinated soon. Great article!