The Pfizer and Moderna Vaccines
In the past weeks, Moderna and Pfizer both announced effective candidates for the COVID-19 vaccine. As cases in the United States continue to spike, the need for a vaccine is increasingly more apparent.
November 21, 2020
After an exhausting eight months stuck in a pandemic and the recent spike of COVID-19 cases, everyone around the nation is desperately awaiting the release of a vaccine. Fortunately, two companies, Pfizer and Moderna, have recently announced promising candidates for the COVID-19 vaccine.
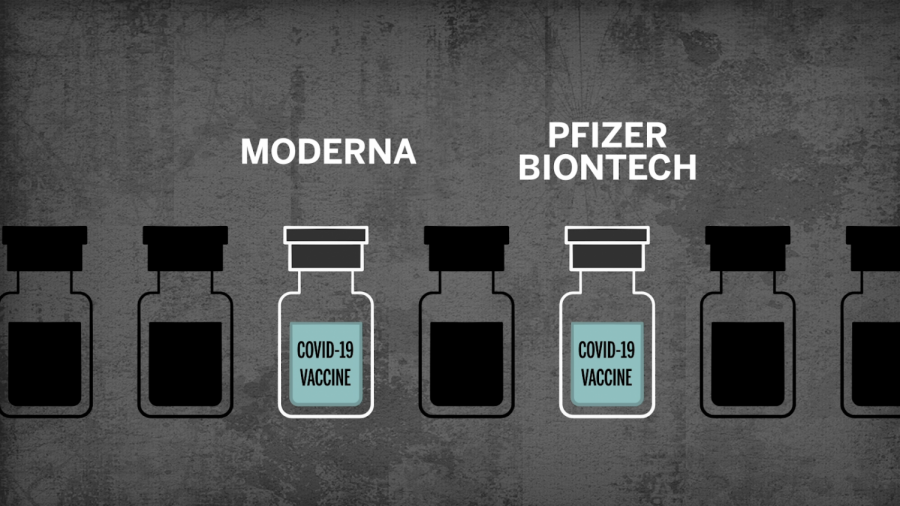
On November 9, 2020, Pfizer and BioNTech announced that their vaccine candidate, BNT162b2, against COVID-19 is more than 90% effective after their first phase III clinical trial. An external and independent Data Monitoring Committee (DMC) reported the percentage and found no adverse effects from the vaccine candidate. The phase III trial began on July 27 and garnered 43,538 participants, and they will continue enrolling individuals for their next phase III trials. According to the current projections, Pfizer is supposed to put out 50 million vaccines in 2020 and 1.3 billion in 2021, but each person needs to take two doses of the vaccine for it to be effective against COVID-19. Dr. Albert Bourla, the CEO and Chairman of Pfizer, believes that with the news regarding the efficacy of the vaccine candidate, they “are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis” (pfizer.com).
On November 16, 2020, a week after the Pfizer announcement, Moderna announced a vaccine that is 94.5% effective after a trial done with around 30,000 participants. Moderna stated in a press conference that there were no significant safety concerns except fatigue and muscle pain. Dr. Stephen Hoge, the president of Moderna, was overjoyed about this “really important milestone” and believes that the news about the vaccines of both Pfizer and Moderna will give the world “hope that a vaccine going to be able to stop this pandemic and hopefully get us back to our lives” (times.com).
Dr. Anthony Fauci, the director of the National Institute of Allergy and Infectious Diseases, was surprised about the efficacy of the vaccines; initially, he would have been happy with an efficacy rate of 70-75%. Both vaccine candidates use mRNA technology or synthetic messenger RNA. In this new vaccine technology, the vaccine creates instructions to fight off diseases. Pfizer and Moderna will soon give their vaccine candidate to the Food and Drug Association (FDA), but the FDA has yet to approve a vaccine using this technology. However, both companies are relatively confident that the FDA will authorize their vaccines (statnews.com).
With the possibility that these vaccines will be approved in the coming weeks, one question remains: who will get the vaccines first? Since there are not enough dosages of the vaccines to be distributed to everyone, the Centers for Disease Control and Infection (CDC) identified four priority groups that will receive the vaccine first: health care workers, essential workers, people with underlying conditions, and older adults. Pfizer’s Senior Vice President of Vaccine Clinical Research and Development, Bill Gruber, stated these priority groups could potentially receive the vaccine in mid-December. For everyone else, Dr. Fauci believes the vaccines will be available for them by April, May, or June 2021 (cnet.com).
Amidst the rise of COVID-19 cases, many students at Yorba Linda High School decided not to return to school and are avoiding hanging out with their friends. The news of these potential vaccines left many YLHS students optimistic. Kylie Chung (11) feels “hopeful” about the news and “wants the vaccine to come out as soon as possible so [she] can go back to school and see [her] friends.” As we await the release of the vaccine, everyone around the world should continue wearing masks and social distancing.


































Kylie de Best • Dec 7, 2020 at 1:18 PM
Thanks for clarifying the information about the differences in both vaccines. It was interesting to see how Fauci thinks about this too, and I am hoping that the vaccine proves effective in stopping the pandemic.
Kayden Mandley • Dec 7, 2020 at 8:34 AM
I really enjoyed this article! It was super informational. Great job Danielle!!
Nikole galea • Nov 30, 2020 at 5:54 PM
Great article! The article allowed me to understand so much about our current situation in overcoming this pandemic without too much of the stuffy sciency words. I think you incorporated the question of who will receive the vaccine first with the information really well. Overall very interesting!
Sharon Sun • Nov 29, 2020 at 9:01 PM
Hey Danielle, great article! I liked your usage of statistics, statements by Dr. Fauci, and explanations about how the vaccines will work. Excited for the vaccines to come out!